Zeroth Law of Thermodynamics
The "zeroth law" states that if two systems are at the same time in thermal equilibrium with a third system, they are in thermal equilibrium with each other.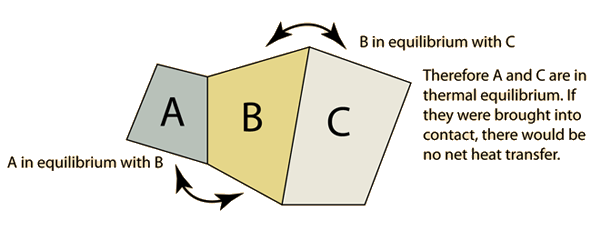
If A and C are in thermal equilibrium with B, then A is in thermal equilibrium with B. Practically this means that all three are at the same temperature, and it forms the basis for comparison of temperatures. It is so named because it logically precedesthe First and Second Laws of Thermodynamics.

0 Comments to "Understanding Thermal Equilibrium"
Leave a Reply